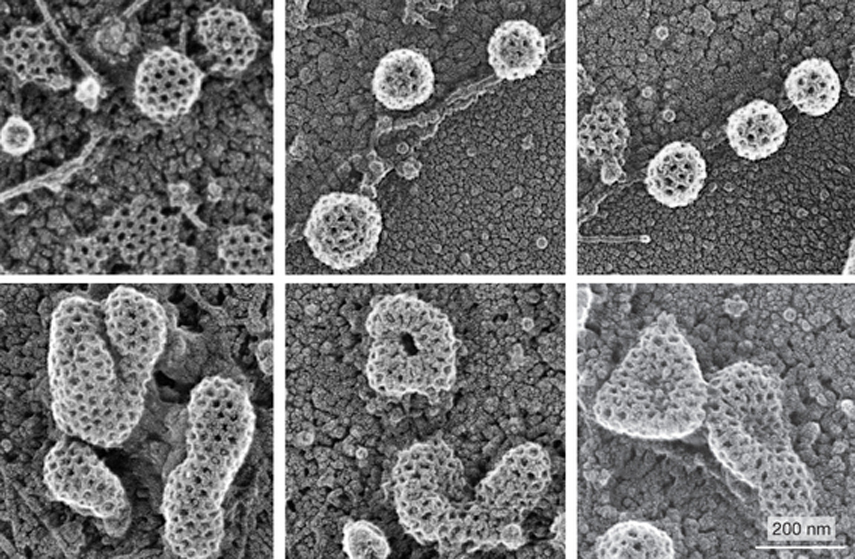
During endocytosis, a cell takes in material from its outside environment. Clathrin-mediated endocytosis involves the inward budding of vesicles, and depends on a lattice, or “coat”, of clathrin molecules that help shape the rounded vesicle. A recent paper describes the results of a screen to find regulators of clathrin-coated vesicle formation at the plasma membrane, one of the earliest steps in clathrin-mediated endocytosis. Kozik and colleagues screened the entire human genome for regulators, and found 92 genes that affect this process. One of these genes encodes the protein V-ATPase, which is found in many membranes and can pump protons across a membrane to regulate pH. In the images above, V-ATPase-inhibited cells (bottom) formed enlarged clathrin-coated structures, compared to the small and uniform clathrin-coated vesicles in control cells (top). Kozik and colleagues found that V-ATPase inhibition blocked the recycling of cholesterol back to the plasma membrane, where it has been suggested that cholesterol aids in membrane bending.
BONUS!! Electron microscopy image of a heart-shaped vesicle, acquired as part of the screen to find clathrin regulators.
Adapted by permission from Macmillan Publishers Ltd, copyright ©2013

No comments:
Post a Comment